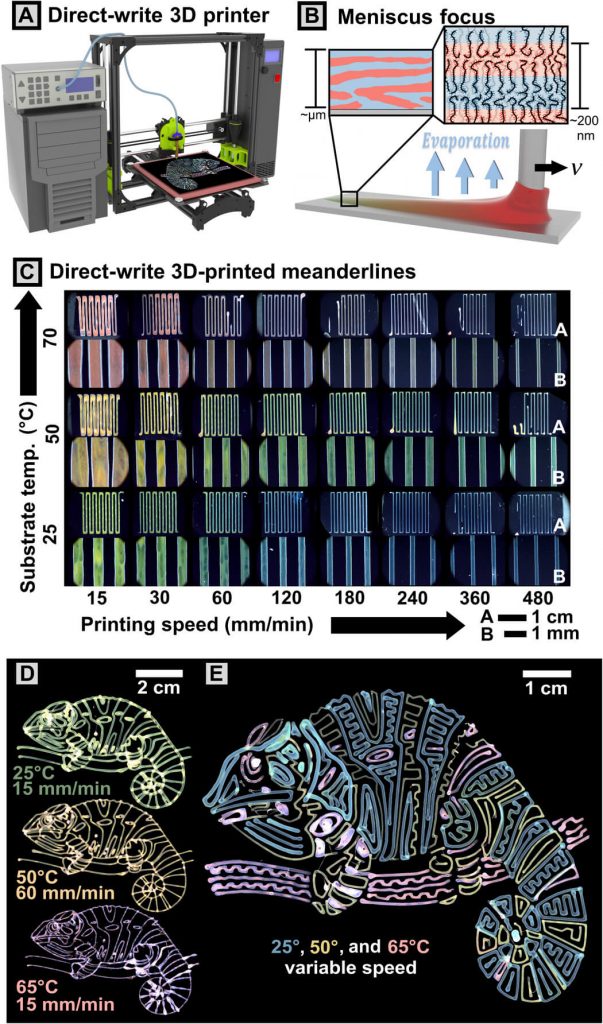
Brilliantly colored chameleons, butterflies and opals reflect color by using nanoscale structures called photonic crystals. A team of US researchers has now taken advantage of the same approach to develop a 3D-printing process that can produce multiple colors from a single ink. They report the new process in a paper in Science Advances.
Some of the most vibrant colors in nature come from a nanoscale phenomenon called structural coloration. When light rays reflect off these periodically placed structures located in the wings and skins of some animals and within some minerals, the rays constructively interfere with each other to amplify certain wavelengths and suppress others. When the structures are well-ordered and small enough – about a thousand times smaller than a human hair – this process causes the rays to produce a vivid burst of color.
“It is challenging to reproduce these vibrant colors in the polymers used to produce items like environmentally friendly paints and highly selective optical filters,” said study leader Ying Diao, a professor of chemical and biomolecular engineering at the University of Illinois at Urbana-Champaign. “Precise control of polymer synthesis and processing is needed to form the incredibly thin, ordered layers that produce the structural color as we see in nature.”
By carefully tuning the assembly process of uniquely structured bottlebrush-shaped polymers during 3D printing, the researchers have found a way to print photonic crystals with tunable layer thicknesses that reflect the visible light spectrum with a single ink.
This ink contains branched polymers with two bonded, chemically distinct segments. The researchers dissolve the material into a solution that bonds the polymer chains just before printing. After printing and as the solution dries, the components separate at a microscopic scale, forming nanoscale layers that exhibit different physical properties depending on the speed of assembly.
“The biggest challenge of the polymer synthesis is combining the precision required for the nanoscale assembly with the production of the large amounts of material necessary for the 3D-printing process,” said co-author Damien Guironnet, also a professor of chemical and biomolecular engineering at the University of Illinois at Urbana-Champaign.
In the lab, the team uses a modified consumer 3D printer to fine-tune how fast the printing nozzle moves across a temperature-controlled surface. “Having control over the speed and temperature of ink deposition allows us to control the speed of assembly and the internal layer thickness at the nanoscale, which a normal 3D printer cannot do,” said Bijal Patel, a graduate student in the Department of Chemical and Biomolecular Engineering at the University of Illinois at Urbana-Champaign and lead author of the paper. “That dictates how light will reflect off of them and, therefore, the color we see.”
The researchers said the color spectrum they have achieved with this method is limited, but they are working to make improvements by learning more about the kinetics behind how the multiple layers form in this process.
Additionally, the team is working on expanding the industrial relevance of the process, as the current method is not well suited for large-volume printing. “We are working with the Damien Guironnet, Charles Sing and Simon Rogers groups at the University of Illinois to develop polymers and printing processes that are easier to control, bringing us closer to matching the vibrant colors produced by nature,” Diao said.
“This work highlights what is achievable as researchers begin to move past focusing on 3D printing as just a way to put down a bulk material in interesting shapes,” Patel said. “Here, we are directly changing the physical properties of the material at the point of printing and unlocking new behavior.”